
(Fentanyl Transdermal Patch)
Therapeutic indication: Fencino® (fentanyl) is a transdermal patch indicated for management of severe chronic pain that requires continuous long-term opioid administration. The Fencino® patch should be replaced every 72 hours.
Adults
Fencino is indicated for management of severe chronic pain that requires continuous long term opioid administration.
Children
Long term management of severe chronic pain in children from 2 years of age who are receiving opioid therapy.
View the Prescribing Information
Adverse events should be reported
Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/.
Adverse events should also be reported to Luye Pharma Ltd at safety@luyepharma.co.uk.
HELPFUL INFORMATION
These videos are promotional in nature and meant for UK HCP’s. Prescribing Information and adverse event reporting is available above.
Below you can see two videos that provide further information for prescribing Fencino® and how to use it, that you and your team may find useful. There is also a short information leaflet about Fencino® that you can download.
1. Overview of Fencino®
Dr Nicholas Herodotu, BSc (Med.Sci. Hons), MB, BS, DGM, DRCOG, DPM, MRCGP, FRCP, FHEA – Palliative Medicine Consultant
Luton and Dunstable University Hospital.
UK-COR-53 June 2024
Video links to external source (Vimeo).
2. Practical Considerations when using Fencino®
Dr Nicholas Herodotu, BSc (Med.Sci. Hons), MB, BS, DGM, DRCOG, DPM, MRCGP, FRCP, FHEA – Palliative Medicine Consultant
Luton and Dunstable University Hospital.
UK-COR-53a June 2024
Video links to external source (Vimeo).
Technical information
| Active Ingredients: | Fentanyl |
| Therapeutic area: | Pain management |
| Pharmaceutical Form: | Transdermal Patch |
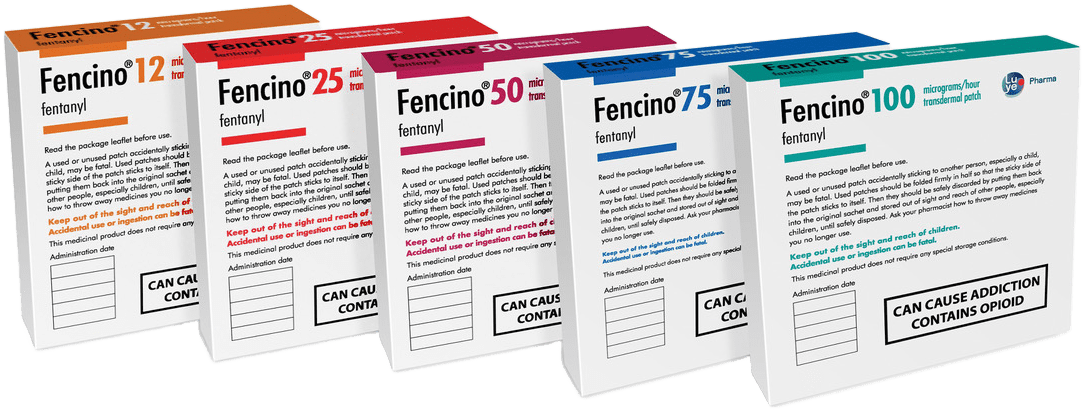
Strengths, Pack size and Information
| Description | Strength | Pack Size | PIP Code |
|---|---|---|---|
| Fencino® | 12mcg | 5 patches | 3649936 |
| Fencino® | 25mcg | 5 patches | 3649977 |
| Fencino® | 50mcg | 5 patches | 3649969 |
| Fencino® | 75mcg | 5 patches | 3649951 |
| Fencino® | 100mcg | 5 patches | 3649944 |
