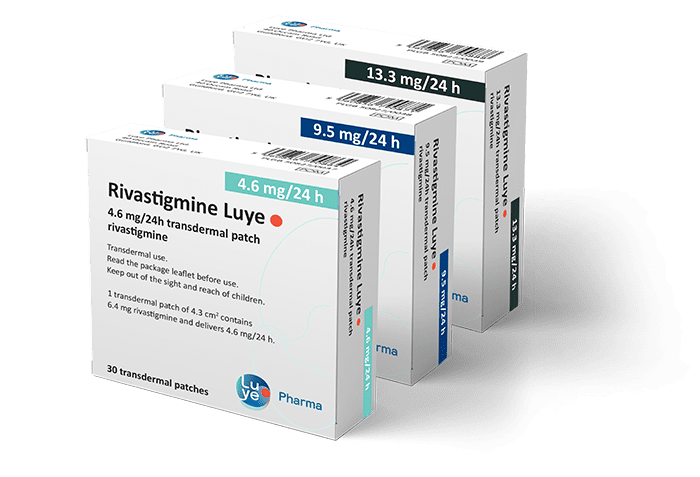
Rivastigmine Luye
(Single Day Transdermal Patch)
Therapeutic indications: Rivastigmine Luye Single Day Transdermal Patch is used for the treatment of adult patients with mild to moderately severe Alzheimer’s dementia, a progressive brain disorder that gradually affects memory, intellectual ability and behaviour. The Rivastigmine Luye Single Day Transdermal Patch should be replaced every 24 hours, the previous patch should be removed before putting one new patch on.
Adverse events should be reported
Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/.
Adverse events should also be reported to Luye Pharma Ltd at safety@luyepharma.co.uk.
This is a Prescription only Medication and is only available as a pharmacy order.
Technical information
| Active Ingredients: | Rivastigmine |
| Therapeutic area: | Neurology |
| Pharmaceutical Form: | Transdermal Patch |
Strengths, Pack size and Information
| Description | Strength | Pack Size | PIP Code |
|---|---|---|---|
| Rivastigmine Luye | 4.6mg/24h | 30 patches | 1279181 |
| Rivastigmine Luye | 9.5mg/24h | 30 patches | 1279199 |
| Rivastigmine Luye | 13.3mg/24h | 30 patches | 1279207 |